CLASS-10 | CHEMICAL REACTION AND EQUATION |MCQ
1. In which of the following equations, the mass is not same on both the sides?
(a) Word equation
(b) Skeletal equation
(c) Balanced equation
(d) Both (a) and (b)
2. Which among the following statement (s) is (are) true? Exposure of silver chloride to sunlight for a long duration turns grey due to
(i) the formation of silver by decomposition of silver chloride
(ii) sublimation of silver chloride
(iii) decomposition of chlorine gas from silver chloride
(iv) oxidation of silver chloride
(a) (i) only
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (iv) only
3. Three beakers labelled as A, B and C each containing 25 mL of water were taken. A small amount of NaOH, anhydrous CuSO4, and NaCl were added to the beakers A, B and C respectively. It was observed that there was an increase in the temperature of the solutions contained in beakers A and B, whereas in case of beaker C, the temperature of the solution falls. Which one of the following statements(s) is (are) correct?
(i) In beakers A and B, exothermic process has occurred.
(ii) In beakers A and B, endothermic process has occurred.
(iii) In beaker C exothermic process has occurred.
(iv) In beaker C endothermic process has occurred.
(a) (i) only
(b) (ii) only
(c) (i) and (iv)
(d) (ii) and (iii)
4. Corrosion of metals can be prevented
(a) by coating the metal surface with a paint.
(b) by applying film of grease and oil on the surface of the metal
(c) by covering the surface of the metal with another metal which is more electropositive
(d) all of these
5. Identify the chemical equation which represents a complete balanced equation for the reaction of barium chloride with sodium sulphate to produce barium sulphate and sodium chloride.
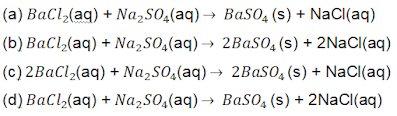
6. Which of the following is not a physical change?
(a) Boiling of water to give water vapour
(b) Melting of ice to give water
(c) Dissolution of alt in water
(d) Combustion of Liquefied Petroleum Gas (LPG)
7. Which among the following is (are) double displacement reaction(s)?
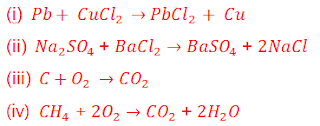
(a) (i) and (iv) (b) (ii) only (c) (i) and (ii) (d) (iii) and (iv)
8. Which of the following reactions are represents a combination reaction?
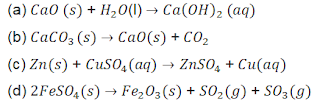
9. Which of the following observation help(s) us to determine that a chemical change has taken place?
(a) Change in temperature.
(b) Change in color
(c) Evolution of a gas.
(d) All of these
10. A dilute ferrous sulphate solution was gradually added to the beaker containing acidified potassium permanganate solution. The light purple colour of the solution fades and finally disappears. Which of the following is the correct explanation for the observation?
(a) KMnO4 is an oxidising agent, it oxidises FeSO4
(b) FeSO4 acts as an oxidising agent and oxidises KMnO4
(c) The color disappears due to dilution, no reaction is involved
(d) KMnO4 is an unstable compound and decomposes in presence of FeSO4 to a colourless compound
11. Which of the following is (are) an endothermic processes?
(1) Dilution of sulphuric acid.
(ii) Sublimation of dry ice.
(iil) Condensation of water vapours.
(iv) Evaporation of water.
(a) (i) and (iii)
(b) (ii) only
(c) (iii) only
(d) (ii) and (iv)
12. Which of the following are combination reactions?
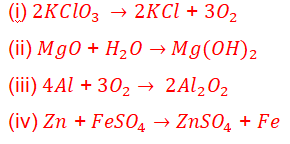
(a) (I) and (iii)
(b) (iii) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
13. In which of the following chemical equations, the abbreviations represent the correct states of the reactants and products involved at reaction temperature?
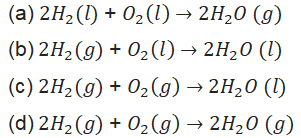
14. Which of the following are exothermic processes?
(i) Reaction of water with quick lime
(ii) Dilution of an acid
(iii) Evaporation of water
(iv) Sublimation of camphor (crystals)
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (iii) and (iv)
15. Which one of the following processes involve chemical reactions?
(a) Storing of oxygen gas under pressure in a gas cylinder
(b) Liquefaction of air
(c) Keeping petrol in a china dish in the open
(d) Heating copper wire in presence of air at high temperature
16. In the double displacement reaction between aqueous potassium iodide and aqueous lead nitrate, a yellow precipitate of lead iodide is formed. While performing the activity if lead nitrate is not available, which of the following can be used in place of lead nitrate?
(a) Lead sulphate (insoluble)
(b) Lead acetate
(c) Ammonium nitrate
(d) Potassium sulphate
17. Solid calcium oxide reacts vigorously with water to form calcium hydroxide accompanied by liberation of heat. This process is called slaking of lime. Calcium hydroxide dissolves in water to form its solution called lime water. Which among the following is (are) true about slaking of lime and the solution formed?
(i) It is an endothermic reaction
(ii) It is an exothermic reaction
(iii) The pH of the resulting solution will be more than seven
(iv) The pH of the resulting solution will be less than seven
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (iii) and (iv)
18. What happens when dilute hydrochloric acid is added to iron filings?
(a) Hydrogen gas and iron (II) chloride are produced
(b) Chlorine gas and ferric hydroxide are produced
(c) Heat is absorbed, i.e., test tubes becomes cold
(d) Iron salt and water are produced
19. The following reaction is used for preparation of oxygen gas in the laboratory:

Which of the following statements is correct about the reaction?
(a) It is a decomposition reaction and endothermic in nature.
(b) It is a combination reaction.
(c) It is a decomposition reaction and is accompanied by release of heat.
(d) It is a photo chemical decomposition reaction and exothermic in nature
20. The following reaction is an example of a
4NH3(8) + 5O2 (e) — 4NO(g)+ 6H2O(g)
(i) displacement reaction
(ii) combination reaction
(iii) redox reaction
(iv) neutralisation reaction
(a) (I) and (iv)
(b) (ii) and (iii)
(c)(i) and (iii)
(d) (iii) and (iv)
21. Chemically rust is
(a) Aluminium sulphate
(b) ferric oxide
(c) hydrated ferric oxide
(d) hydrated ferrous oxide
22. The chemical formula of lead sulphate is
(a) Pb2SO4
(b) Pb(SO4)2
(c) PbSO4
(d) Pb2(SO4)3
23. Both CO2 and H2 gases are
(a) heavier than air
(b) acidic in nature
(c) colourless
(d) soluble in water
24. Which of the following gases can be used for storage of fresh sample of an oil for a long time
(a) Carbon dioxide or oxygen
(b) Nitrogen or helium
(c) Helium or oxygen
(d) Nitrogen or oxygen
25. The electrolytic decomposition of water gives H, and 0, in the ratio of
(a) 2:1 by volume
(b) 1:2 by volume
(c) 8:1 by mass
(d) 1:2 by mass
26. In the decomposition of lead (II) nitrate to give lead (II) oxide, nitrogen dioxide and gas, the coefficient of nitrogen dioxide (in the balanced equation) is
(a) 1
(b) 2
(c) 3
(d) 4
27. Fatty foods become rancid due to the process of
(a) reduction
(b) corrosion
(c) oxidation
(d) hydrogenation
28. We store silver chloride in a dark coloured bottle because it
(a) is a white solid
(b) undergoes redox reaction
(c) decomposes by sunlight
(d) none of the above
29. Silver article turns black when kept in the open for a few days due to formation of
(a) H2S
(b) AgS
(c) AgSO2
(d) Ag2S
30. When crystals of lead nitrate are heated strongly in a dry test tube
(a) crystals immediately melt
(b) a brown residue is left
(c) white fumes appear in the tube
(d) a yellow residue is left
31. Dilute hydrochloric acid is added to granulated zinc taken in a test tube. The following observations are recorded. Point out the correct observation.
(a) The surface of metal becomes shining
(b) The reaction mixture turns milky
(c) Odour of a pungent smelling gas is recorded
(d) A colourless and odourless gas is evolved
32. When carbon dioxide is passed through lime water,
(a) calcium hydroxide is formed
(b) white precipitate of CaO is formed
(c) lime water turns milky
(d) colour of lime water disappears.
33. When a magnesium ribbon is burrnt in air, the ash formed is
(a) black
(b) white
(c) yellow
(d) pink
34. In which of the following, heat energy will be evolved?
(a) Burning of L.P.G.
(b) Dissolution of NH4Cl in water
(c) Electrolysis of water
(d) Decomposition of AgBr in the presence of sunlight
35. Rancidity can be prevented by
(a) adding antioxidants
(b) storing food away from light
(c) keeping food in refrigerator
(d) all of these
36. The reaction of H2 gas with oxygen gas to form water is an example of
(a) combination reaction
(b) redox reaction
(c) exothermic reaction
(d) all of these reactions
37. The reaction in which two compounds exchange their ions to form two new compounds is called
(a) displacement reaction
(b) combination reaction
(c) double displacement reaction
(d) redox reaction
38.On immersing an iron nail in CuSO4 solution for few minutes, you will observe
(a) no reaction takes place
(b) the colour of solution changes to green
(c) the surface of iron nails acquire a black coating
(d) the colour of solution fades
39. An element X on exposure to moist air turns reddish-brown and a new compound Y is formed. The substance X and Y are
(a) X = Fe, Y = Fe2O3
(b) X = Ag, Y = Ag2S
(c) X = Cu, Y = CuO
(d) X = Al, Y = Al2O3
40. Which of the following is termed as oxidizing agent?
(a) Which gives Oxygen
(b) Which removes oxygen
(c) Which gives hydrogen
(d) All of the above
41. Which of the following termed as reducing agent?
(a) Which gives oxygen
(b) Which removes oxygen
(c) Which removes hydrogen
(d) All of the above
42.Which of the following does show oxidation reaction?
(a) Gain of oxygen
(b) Loss of oxygen
(c) Gain of hydrogen
(d) None of the above
43. Which of the following does show reduction reaction?
(a) Gain of oxygen
(b) Loss of oxygen
(c) Loss of hydrogen
(d) None of the above
44. Which of the reaction is used in black and white photography?
(a) Combination reaction
(b) Decomposition reaction
(c) Displacement reaction
(d) Oxidation reaction
45. In which of the following heat is evolved?
(a) Combination reaction
(b) Decomposition reaction
(c) Displacement reaction
(d) Double displacement reaction
46. In which of the following heat is absorbed generally?
(a) Combination reaction
(b) Decomposition reaction
(c) Displacement reaction
(d) Double displacement reaction
47. Which of the following is termed as endothermic reaction?
(a) Reaction in which heat is evolved
(b) Reaction in which heat is absorbed
(c) Reaction in which there is loss of oxygen
(d) Reaction in which there is gain of hydrogen
48. Which of the following is termed as exothermic reaction?
(a) Reaction in which heat is evolved
(b) Reaction in which heat is absorbed
(c) Reaction in which there is loss of oxygen
(d) Reaction in which there is gain of hydrogen
49. What is the name of reaction which decomposes after supply of heat?
(a) Combination reaction
(b) Thermal decomposition
(c) Displacement reaction
(d) Redox reaction
50. What is the name of reaction in which both oxidation and reduction takes place?
(a) Combination reaction
(b) Thermal decomposition
(c) Displacement reaction
(d) Redox reaction
51. What is the name of substance which does get deposited over iron because of moisture present in air?
(a) Sulphide
(b) Rust
(c) Carbonate
(d) Oxygen
52. Why magnesium ribbon is cleaned before burning?
(a) To remove dust
(b) To remove magnesium oxide
(c) To remove magnesium
(d) All of the above
53. On the basis of evolution or absorption of heat, chemical reactions can be divided into how many types?
(a) Two
(b) Three
(c) Four
(d) One
54. Which of the following gas is produced when carbon is burnt in air?
(a) Carbon dioxide
(b) Sulphur dioxide
(c) Oxygen
(d) Hydrogen
55. What happens when hydrogen reacts with oxygen?
(a) Carbon dioxide is formed
(b) Water is formed
(c) Hydrogen carbonate is formed
(d) All of the above
56. Which of the following product is formed when calcium oxide reacts with water?
(a) Slaked lime
(b) Carbon dioxide
(c) Calcium oxide
(d) Oxygen gas
57. What is another name of quick lime?
(a) Calcium hydroxide
(b) Calcium oxide
(c) Carbon dioxide
(d) Sodium oxide
58. What is the chemical name for slaked lime?
(a) Calcium carbonate
(b) Calcium oxide
(c) Calcium hydroxide
(d) Carbon monoxide
59. Heating of ferrous sulphate gives which of the following product?
(a) Ferric oxide
(b) Sulphur dioxide
(c) Sulphur trioxide
(d) All of the above
60. In which of the following category will you put the reaction of heating of ferrous sulphate:
(a) Decomposition reaction
(b) Combination reaction
(c) Displacement reaction
(d) All of the above
61. In which of the following category will you put the reaction of heating of calcium carbonate?
(a) Decomposition reaction
(b) Thermal decomposition reaction
(c) Endothermic reaction
(d) All of the above
62. Which of the following product is formed after heating of limestone?
(a) Calcium oxide
(b) Calcium carbonate
(c) Hydrogen gas
(d) All of the above
63. What happens when silver chloride is put under sunlight
(a) Silver metal and chlorine gas are formed
(b) Silver metal and hydrogen gas are formed
(c) Only silver metal is formed
(d) Only hydrogen gas is formed
64. Which of the following is formed when lead nitrate is put under thermal decomposition?
(a) Lead oxide
(b) Nitrogen dioxide
(c) Oxygen gas
(d) All of the above
65. What happens when carbon dioxide is passed through lime water?
(a) Lime water turns milky because of formation of calcium carbonate
(b) Lime water turns milky because of formation of water
(c) Lime water turns red because of formation of permanganate
(d) Lime water turns red because of formation of copper sulphate
66.What happens when silver bromide is exposed to sunlight?
(a) Hydrogen gas is formed
(b) Bromine gas is formed
(c) Chlorine gas is formed
(d) Iodine gas is formed
67. Which metal is displaced when lead is put in the solution of copper chloride?
(a) Lead
(b) Copper
(c) Chlorine
(d) All of the above.
68.Which of the following is formed when lead metal reacts with the solution of copper chloride?
(a) No reaction takes place
(b) Chlorine gas
(c) Lead chloride
(d) Copper-lead complex
69.Which metal is displaced when zinc metal is put in the solution of copper sulphate
(a) Copper
(b) Zinc
(c) Sulphate
(d) All of the above
70. What happens when sodium sulphate solution is mixed with the solution of barium chloride?
(a) Barium sulphate is formed
(b) Sodium sulphate is formed
(c) Sulphur dioxide gas is formed
(d) No reaction takes place
71.What happens when copper metal is dipped in the solution of zinc sulphate?
(a) Copper sulphate is formed
(b) Oxygen gas is formed
(c) Zinc metal is separated
(d) No reaction takes place
72. What happens when zine metal is dipped in the solution of copper sulphate?
(a) Zinc sulphate is formed
(b) Zinc oxide is formed
(c) Zinc sulphide is formed
(d) No reaction takes place
73. Why are articles made of iron painted to prevent rust?
(a) Paint makes articles made of iron beautiful
(b) Paint prevents iron articles to come in contact with moisture present in air.
(c) Paint prevents iron articles from getting sticky
(d) All of the above
74. Which of the following is termed as rancidity?
(a) Reduction of oxygen present in food
(b) Oxidation of oil present in food
(c) Oxidation of sugar present in food
(d) All of the above
75 How rancidity can be prevented?
(a) By adding antioxidants in food
(b) By adding more oxygen to food
(c) By keeping food items in open
(d) All of the above
76. Balanced equation of Fe + H2O → Fe3O4 + H2 is
(a) Fe + 4H2O → Fe3O4+ H2
(b) Fe + 4H2O → Fe3+ 4H2
(c) 3Fe + H2O → Fe3O4+ 4H2
(d) 3Fe + 4H2O → Fe3O4 + 4H2
77. The formula of quick lime and the compound formed when it reacts with water
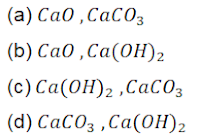
78. A solution of which of the following compounds is used for whitewashing?
(a) Slaked line
(b) Quick Lime
(c) Blue vitriol
(d) Limestone
79. Electrolysis of water is
(a) Combination reaction
(b) Decomposition reaction
(c) Displacement reaction
(d) None
80. Example of displacement reaction is
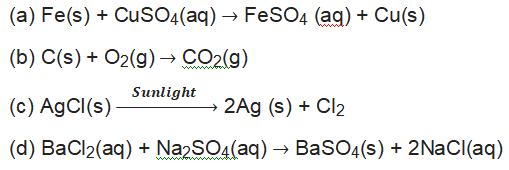
81. Lead nitrate Pb(NO3)2 on heating forms solid Lead oxide (PbO) and Nitrogen dioxide What is the colour of lead oxide and nitrogen dioxide?
(a) White, Colourless
(b) White, Brown
(c) Yellow. Brown
(d) Yellow. Colourless
82. Silver chloride on exposure to sunlight decomposes into silver and chlorine gas. This is used in
(a) Heat production as enormous energy is released
(b) Silver extraction as earth contains silver mainly in silver chloride form.
(c) Photography as grey silver formed produces an image print
(d) None
83. Quick lime is used in white washing because
(a) it is cheap
(b) it forms slaked lime with water which has a nice colour.
(c) it forms slaked lime with water which reacts with atmospheric carbon dioxide to for limestone
(d) None
84. Example of a double displacement reaction will be
(a) Metal with a salt
(b) Metal with an acid
(c) Metal with metal
(d) Salt with a salt
85. Ferrous sulphate solution is green while ferric oxide formed by its decomposition is
(a) red
(b) white
(c) brown
(d) yellow
86. Corrosion or rusting of iron metal is
(a) Oxidation of iron
(b) Reduction of iron
(c) Displacement of iron
(d) None
87. To facilitate the electrolysis of water we add a few drops of acids like salphuric acid because
(a) It acts as a catalyst
(b) It prevents the decomposition of Electrodes used.
(c) It makes the water a good conductor of electricity
(d) None
88. Fried foods are packed with which gas to prevent Oxidation of fat?
(a) Oxygen
(b) Nitrogen
(c) Any of the above
(d) None
89. Which of the following statements about the reaction are correct?
3Fe (s) + 4H2O(g) → Fe3O4(s) + 4H3(g)
(i) Iren metal is getting oxidised
(ii) Water is getting reduced
(iii) Water is acting as reducing agent
(iv) Water is acting as oxidising agent
(a) (i),(ii) and (iii)
(b) (iii) and (iv)
(c) (i) (ii) and (iv)
(d) (ii)and (iv)
90. Which of the following is a displacement reaction?
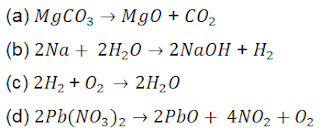
91. A substance X’is used in white-washing and is obtained by beating time stone in the absence of air. The substance ‘X’ is
(a) CaOCl2
(b) Ca(OH)2
(c) CaO
(d) CaCO3
92. Select the oxidising agent for the following reaction:
H2S + I2 → 2HI + S
(a) I2
(b) H2S
(c) HI
(d) S
93. A substance added to food containing fats and oils is called:
(a) Oxidant
(b) Rancid
(c) Coolant
(d) Antioxidant
94. The condition produced by serial oxidation of fats and oils in foods marked by unpleasant smell and taste is called:
(a) antioxidation
(b) reduction
(c) rancidity
(d) corrosion
95. When SO2, gas is passed through saturated solution of H2S which of the following reaction occurs?
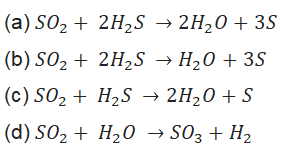
96. What are the products formed when iron filings are heated with dilute hydrochloric acid?
(a) Fe (III) chloride and water
(b) Fe (II) chloride and water
(c) Fe (II) chloride and hydrogen gas
(d) Fe (III) chloride and hydrogen gas
97. A student poured 100 mL of water in a bottle and added 40 mL vinegar to it. A balloon was filled with 20 g baking soda and was fixed at the mouth of the bottle. Slowly the shape of the balloon changed, as shown below:
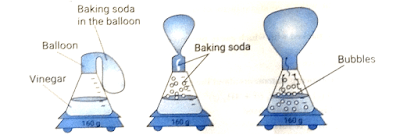
The student claims that a chemical change happened when the two substances were mixed. Is the claim made by the student correct?
(a) Yes, as a new substance was formed in the form of a gas.
(b) No, as the formation of bubbles in the mixture shows a physical change.
(c) Yes, as the mass remains the same throughout the experiment.
(d) No, as the change in the shape and size of the balloon shows a physical change.
98. A student performs an experiment to form aluminium chloride from aluminium and chlorine. Which options gives the balanced chemical equation of the reaction?
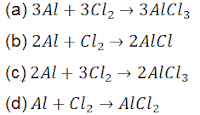
99. A researcher adds barium hydroxide to hydrochloric acid to form a white coloured barium chloride. Which option gives the balanced chemical equation of the reaction
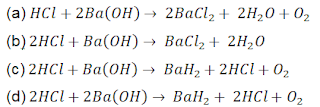
100. A student writes a balanced chemical equation as
Pb(s) + CuCl2(aq) → PbCl2(aq) + Cu(s)
Which option gives the correct type of chemical reaction?
(a)Combination Reaction
(b) Decomposition Reaction
(c) Displacement reaction
(d)Double displacement reaction
| ANSWERS | |||||||||
| 1(d) | 2(a) | 3(c) | 4(d) | 5(d) | 6(d) | 7(b) | 8(a) | 9(d) | 10(a) |
| 11(d) | 12(c) | 13(d) | 14(a) | 15(d) | 16(b) | 17(b) | 18(a) | 19(a) | 20(c) |
| 21(c) | 22(c) | 23(c) | 24(b) | 25(a) | 26(d) | 27(c) | 28(c) | 29(d) | 30(d) |
| 31(d) | 32(c) | 33(b) | 34(a) | 35(d) | 36(d) | 37(c) | 38(b) | 39(a) | 40(a) |
| 41(b) | 42(a) | 43(b) | 44(b) | 45(a) | 46(b) | 47(b) | 48(a) | 49(b) | 50(d) |
| 51(b) | 52(b) | 53(a) | 54(a) | 55(b) | 56(a) | 57(b) | 58(c) | 59(d) | 60(a) |
| 61(d) | 62(a) | 63(a) | 64(d) | 65(a) | 66(b) | 67(b) | 68(c) | 69(a) | 70(a) |
| 71(d) | 72(a) | 73(b) | 74(b) | 75(a) | 76(d) | 77(b) | 78(b) | 79(b) | 80(a) |
| 81(c) | 82(c) | 83(c) | 84(d) | 85(c) | 86(a) | 87(c) | 88(b) | 89(c) | 90(b) |
| 91(c) | 92(a) | 93(d) | 94(c) | 95(a) | 96(c) | 97(a) | 98(c) | 99(b) | 100(c) |