CLASS-10 | ACIDS BASES AND SALTS | CBSE BOARD
100 MULTIPLE CHOICE QUESTIONS FROM ACIDS BASES AND SALTS
1. Calcium carbonate is the chemical formula of
(a) limestone
(b) chalk
(c) marble
(d) all (a), (b) and (c)
Answer:
(d) all (a), (b) and (c)
2. On adding dilute HCl to copper oxide in a beaker, the solution turns blue-green due to formation of
(a) copper(II) hydroxide
(b) copper nitrate
(c) copper (II) chloride
(d) copper sulphate
Answer:
(c) copper (II) chloride
3. Human body works within the pH range of
(a) 7.0 to 7.8
(b) 1.5 to 5.6
(c) 13.0 to 14.0
(d) 1.2 to 2.2
Answer:
(a) 7.0 to 7.8
4. A basic solution could have a pH of
(a) 1
(b) 11
(c) 7
(d) 2
(b) 11
5. Which of the following gives the correct increasing order of acidic strength?
(a) Water < Acetic acid < Hydrochloric acid
(b) Water < Hydrochloric acid < Acetic acid
(c) Acetic acid < Water < Hydrochloric acid
(d) Hydrochloric acid < Water < Acetic acid
Answer:
(a) Water < Acetic acid < Hydrochloric acid
6. Fruit juices, such as orange juice, contain
(a) boric acid
(b) citric acid
(c) sulphuric acid
(d) nitric acid
Answer:
(b) citric acid
7. Common salt, besides being used in kitchen, can also be used as the raw material for making
(i) washing soda (ii) bleaching powder
(iii) baking soda (iv) slaked lime
(a) (i) and (ii)
(b) (i) ,(ii) and (iv)
(c) (i) and (iii)
(d) (i) ,(iii) and (iv)
Answer:
(c) (i) and (iii)
8. Which of the following salts does not contain water of crystallisation?
(a) Blue vitriol
(b) Baking soda
(c) Washing soda
(d) Gypsum
Answer:
(b) Baking soda
9. A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange. Which of the following would change the colour of this pH paper to greenish-blue?
(a) Lemon juice
(b) Vinegar
(c) Common salt
(d) An antacid
Answer:
(d) An antacid
10.In an attempt to demonstrate electrical conductivity through an electrolyte, the alongside apparatus was set up. Which among the following statement(s) is /are correct?
(i) Bulb will not glow because electrolyte is not acidic
(ii) Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
(iii) Bulb will not glow because circuit is incomplete.
(iv) Bulb will not glow because it depends upon the type of electrolytic solution.
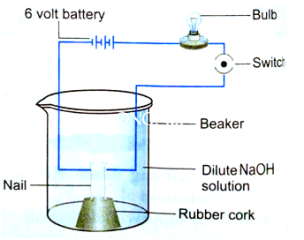
(a) (i) and (iii)
(b) (ii) and(iv)
(c) (ii) only
(d) (iv) only
Answer:
(c) (ii) only
11. Which of the following solutions will turn phenolphthalein pink?
(a) HCl (aq)
(b) CO2(aq)
(c) KOH(aq)
(d) H2SO4(aq)
Answer:
(c) KOH(aq)
12. Identify the correct representation of reaction occurring during chlor-alkali process.
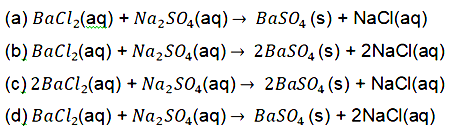
Answer:(d)
13. Which of the following statements is true for acids?
(a) Bitter and change red litmus to blue
(b) Sour and change red litmus to blue
(c) Sour and change blue litmus to red
(d) Bitter and change blue litmus to red
Answer:
(c) Sour and change blue litmus to red
14. The acid having highest hydrogen ion concentration is one with
(a) pH = 2.5
(b) pH = 1.8
(c) pH = 7
(d) pH = 10
Answer:
(b) pH = 1.8
15. The pH of the gastric juices released during digestion is:
(a) less than 7
(b) more than 7
(c) equal to 7
(d) equal to 0
Answer:
(a) less than 7
16. If a few drops of a concentrated acid accidentally spills over the hand of a student, what should be done?
(a) Wash the hand with saline solution.
(b) Wash the hand immediately with plenty of water and apply a paste of sodium hydrogen carbonate.
(c) After washing with plenty of water, apply solution of sodium hydroxide on the hand.
(d) Neutralise the acid with a strong alkali
Answer:
(b) Wash the hand immediately with plenty of water and apply a paste of sodium hydrogen carbonate.
17. Sodium hydrogen carbonate when added to acetic acid evolves a gas. Which of the following statements is true about the gas evolved?
(i) It turns lime water milky.
(ii) It extinguishes a burning splinter.
(iii) It dissolves in a solution of sodium hydroxide.
(iv) It has a pungent odour.
(a) (i) and (ii)
(b) (i), (ii) and (iii)
(c) (ii). (iii) and (iv)
(d) (i) and (iv)
Answer:
(b) (i), (ii) and (iii)
18. Which solution will change blue litmus to red?
(a) NaOH(aq)
(b) H2SO4(aq)
(c) KCl(aq)
(d) NH4OH(aq)
Answer:
(b) H2SO4(aq)
19. Which is a soluble base in water?
(a) Cu(OH)2
(b) Fe(OH)3
(c) Zn(OH)2
(d) KOH
(d) KOH
20. In general, salts
(a) are ionic compounds.
(b) contain hydrogen ions.
(c) contain hydroxide ions.
(d) turn blue litmus red.
Answer:
(a) are ionic compounds.
21. Which of the following properties is closely related to acids?
(a) Contain the hydroxide ion
(b) Bitter taste
(c) Salty taste
(d) Sour taste
Answer:
(d) Sour taste
22.A base can be prepared by the reaction between
(a) an active non-metal and water.
(b) a gas and water
(c) a sulphide and water.
(d) an active metal and water.
Answer:
(d) an active metal and water.
23.Which of the following is (are) true when HCI(g) is passed through water?
(i) It does not ionise in the solution as it is a covalent compound.
(ii) It ionises in the solution.
(iii) It gives both hydrogen and hydroxyl ion in the solution.
(iv) It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule.
(a) (i) only
(b) (iii) only
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer:
(c) (ii) and (iv)
24. What happens when a solution of an acid is mixed with a solution of a base in a test tube?
(i) Temperature of the solution decreases
(ii) Temperature of the solution increases
(iii) Temperature of the solution remains the same
(iv) Salt formation takes place
(a) (i) and (iv)
(b) (i) and (ii)
(c) (i) only
(d) (ii) and (iv)
Answer:
(d) (ii) and (iv)
25. When hydrogen chloride gas is prepared on a humid day, the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to
(a) absorb the evolved gas
(b) moisten the gas
(c) absorb moisture from the gas
(d) absorb CI ions from the evolved gas
Answer:
(c) absorb moisture from the gas
26. What is formed when zinc reacts with sodium hydroxide?
(a) Zinc hydroxide and sodium
(b) Sodium zincate and hydrogen gas
(c) Sodium zinc-oxide and hydrogen gas
(d) Sodium zincate and water
Answer:
(b) Sodium zincate and hydrogen gas
27. Tomato is a natural source of which acid?
(a) Acetic acid
(b) Citric acid
(c) Tartaric acid
(d) Oxalic acid
Answer:
(d) Oxalic acid
28. Brine is an
(a) aqueous solution of sodium hydroxide
(b) aqueous solution of sodium carbonate
(c) aqueous solution of sodium chloride
(d) aqueous solution of sodium bicarbonate
Answer:
(c) aqueous solution of sodium chloride
29. Na2CO3.10H2O is
(a) washing soda
(b) baking soda
(c) bleaching powder
(d) tartaric acid
Answer:
(b) baking soda
30. At what temperature is gypsum heated to form Plaster of Paris?
(a) 90°C
(b) 100°C
(c) 110°C
(d) 120°C
Answer:
(b) 100°C
31. How many water molecules does hydrated calcium sulphate contain?
(a) 5
(b) 10
(c) 7
(d) 2
Answer:
(d) 2
32. Sodium carbonate is a basic salt because it is a salt of
(a) strong acid and strong base
(b) weak acid and weak base
(c) strong acid and weak base
(d) weak acid and strong base
Answer:
(d) weak acid and strong base
33. Alkalis are
(a) acids, which are soluble in water
(b) acids, which are insoluble in water
(c) bases, which are insoluble in water
(d) bases, which are soluble in water
Answer:
(d) bases, which are soluble in water
34. Which of the following statements is correct about an aqueous solution of an acid and oral base?
(i) Higher the pH, stronger the acid
(ii) Higher the pH, weaker the acid
(iii) Lower the pH, stronger the base
(iv) Lower the pH, weaker the base
(a) (i) and (iii)
(b) (ii) and (iii)
(c)(i) and (iv)
(d) (ii) and (iv)
Answer:
(d) (ii) and (iv)
35. The apparatus given in the adjoining figure was set up to demonstrate electrical conductivity Which of the following statement(s) is Bulb (are) correct?
(i) Bulb will not glow because electrolyte is not acidic.
(ii) Bulb will glow because HCI is a strong acid and furnishes ions for conduction.
(iii) Bulb will not glow because circuit is Nail incomplete.
(iv) Bulb will not glow because it depends upon the type of electrolytic Rubber cork solution.
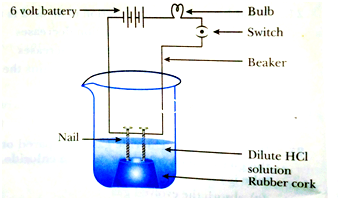
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (ii) only
(d) (iv) only
Answer:
(c) (ii) only
36. Lime water reacts with chlorine to give
(a) bleaching powder
(b) baking powder
(c) baking soda
(d) washing soda
Answer:
(a) bleaching powder
37. Nettle sting is a natural source of which acid?
(b) Lactic acid
(c) Citric acid
(d) Tartaric acid
Answer:
(a) Methanoic acid
38. Tooth enamel is made up of
(a) calcium phosphate
(b) calcium carbonate
(c) calcium oxide
(d) potassium
Answer:
(a) calcium phosphate
39. Rain is called acid rain when its
(a) pH falls below 7
(b) pH falls below 6
(c) pH falls below 5.6
(d) pH is above 7
Answer:
(c) pH falls below 5.6
40. Sodium hydroxide is a
(a) weak base
(b) wear acid
(c) strong base
(d) strong acid
Answer:
(c) strong base
41. An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?
(a) Baking powder
(b) Lime
(c) Ammonium hydroxide solution
(d) Hydrochloric acid
Answer:
(d) Hydrochloric acid
42.When copper oxide and dilute hydrochloric acid react, colour changes to
(a) white
(b) bluish-green
(c) blue-black
(d) black
Answer:
(b) bluish-green
43. Sodium hydroxide is used
(a) as an antacid
(b) in manufacture of soap
(c) as a cleansing agent
(d) in alkaline batteries
Answer:
(b) in manufacture of soap
44. Sodium hydroxide turns phenolphthalein solution
(a) pink
(b) Yellow
(c) colourless
(d) orange
Answer:
(a) pink
45. Chemical formula of washing soda is
(a) Na2CO3.7H2O
(b) NaCO3.5H2O
(c) Na2CO3.2H2O
(d) Na2CO3.10H2O
Answer:
(d) Na2CO3.10H2O
46.Which of the following is not a acidic salt?
(a) CuSO4
(b) NH4Cl
(c) FeCl3
(d) CH3COONa
Answer:
(d) CH3COONa
47. A solution of NaCl
(a) will turn red litmus blue
(b) will turn pH paper green
(c) will turn blue litmus red
(d) will not affect litmus
Answer:
(d) will not affect litmus
48. Many salts absorbs water from atmosphere. This property is called
(a) deliquescence
(b) efflorescence
(c) hydration
(d) addition
Answer:
(a) deliquescence
49. An aqueous solution with pH = 1 is
(a) strongly acidic
(b) strongly basic
(c) neutral
(d) weakly acidic
Answer:
(a) strongly acidic
50. CaOCl2 will liberate Cl2 gas in presence of
(i) CO2 (ii) HCI (iii) CO (iv) NO
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer:
(a) (i) and (ii)
51. Egg shell is made up of.
(a) CaCO3
(b) CaO
(c) Ca(OH)2
(d) CaCl2
52. Curd cannot be stored in
(i) Brass vessel
(ii) Steel
(iii) Copper vessel
(iv) Bronze
(a) (I),(ii),(iii)
(b) (ii),(iii),(iv)
(c) (i),(ii),(iv)
(d) (I),(iii),(iv)
Answer:
(d) (I),(iii),(iv)
53. Sodium carbonate is a basic salt because it is a salt of
(a) strong acid and strong base.
(b) weak acid and weak base.
(c) strong acid and weak base.
(d) weak acid and strong base.
Answer:
(d) weak acid and strong base.
54. Calcium phosphate is present in tooth enamel. Its nature is
(a) basic
(b) acidic
(c) neutral
(d) amphoteric
Answer:
(a) basic
55. Which one of the following can be used as an acid-base indicator by a visually impaired (blind) student
(a) Litmus
(b) Turmeric
(c) Vanilla essence
(d) Petunia leaves
Answer:
(c) Vanilla essence
56. Which of the following is used for dissolution of gold?
(a) Hydrochloric acid
(b) Sulphuric acid
(c) Nitric acid
(d) Aqua regia
Answer:
(d) Aqua regia
57. Which of the following are present in a dilute aqueous solution of hydrochloric acid?
(a) H3O++ Cl–
(b) H3O++ OH–
(c) Cl– + OH–
(d) unionised HCI
Answer:
(a) H3O++ Cl–
58. NaHCO3, formed by reaction of
(a) NaOH + H2CO3
(b) NaCl + H2CO3
(c) Na2CO3+ H2S
(d) NaOH + Na2CO3
Answer:
(d) NaOH + Na2CO3
59. pH of H2O is
(a) 7
(b) 8
(c) 9
(d) 10
Answer:
(a) 7
60. Ag2S reacts with H2SO4 to form
(a) AgSO4
(b) Ag2SO4 + H2S
(c) Ag2O + H2S
(d) AgOH + H2S
61. Lime water reacts with chlorine to form
(a) CaCl2
(b) CaOCl2
(c) Ca(ClO3)2
(d) CaO2Cl2
Answer:
(b) CaOCl2
62. NaOH is obtained by electrolysis of
(a) Aq solution of NaCl
(b) Aq. Na2CO3
(c) Aq. NaHCO3
(d) Molten NaCl
Answer:
(d) Molten NaCl
63. The chemical name of bleaching powder is
(a) calcium hypo oxychloride
(b) calcium oxychloride
(c) calcium chloride
(d) calcium chloro oxide
Answer:
(b) calcium oxychloride
64. The ratio of the water molecule in Plaster of Paris and Gypsum is
(a) 3:1
(b) 1:3
(c) 1:4
(d) 4:3
Answer:
(c) 1:4
65. Baking powder is
(a) sodium carbonate + sodium tartarate
(b) sodium bicarbonate + sodium tartarate
(c) sodium bicarbonate + tartaric acid
(d) sodium carbonate + sodium benzoate
Answer:
(c) sodium bicarbonate + tartaric acid
66. Gastric juice contains HCl which is one example of
(a) inorganic acid
(b) organic acid
(c) soft organic acid
(d) strong inorganic acid
Answer:
(a) inorganic acid
67. When milk of magnesia reacts with acetic acid it produces
(a) basic salt
(b) acidic salt
(c) neutral salt
(d) complex salt
Answer:
(c) neutral salt
68. Which of the following phenomena will occur when a small amount of acid is added to water?
(i) dilution (ii) neutralization (iii) salt formation (iv) ionization
(a) (i) and (ii)
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer:
(b) (i) and (iv)
69. Brine is used for industrial production of
(a) NaOH
(b) КОН
(c) bleaching powder
(d) none of the above
Answer:
(a) NaOH
70. When base reacts with the non-metal oxide
(a) it neutralizes each other
(b) it creates fire
(c) it produces acidic salts
(d) it produces basic salts
Answer:
(a) it neutralizes each other
71.Corrosive effect on the skin is caused by
(a) acids and bases
(b) bases and salt
(c) water
(d) mercury
Answer:
(a) acids and bases
72. The acid used for the manufacture of fertilizers and explosives is
(a) nitric acid
(b) sulphuric acid
(c) phosphoric acid
(d) hydrochloric acid
Answer:
(a) nitric acid
73. Which acid is found in bee sting?
(a) Citric acid
(b) Formic acid
(c) Tartaric acid
(d) Nitric acid
Answer:
(b) Formic acid
74. Rubbing of which does give relief from pain in the case of bee sting?
(a) Dilute hydrochloric acid
(b) Dilute nitric acid
(c) Tooth paste
(d) Alkali
Answer:
(c) Tooth paste
75. Which statement is correct?
(a) Organic acids are obtained from natural sources.
(b) Inorganic acids are prepared in laboratory.
(c) Bee sting contains formic acid.
(d) All of the above.
Answer:
(d) All of the above.
76. What happens when acid is mixed with water?
(a) Heat is evolved
(b) Heat is absorbed
(c) Concentration of acid increases
(d) All of the above
Answer:
(a) Heat is evolved
77. What happens when an alkali is mixed with water?
(a) Heat is evolved
(b) Heat is absorbed
(c) Concentration of acid increases
(d) All of the above
Answer:
(a) Heat is evolved
78. Which of the following is alkali?
(a) Sodium hydroxide
(b) Calcium carbonate
(c) Copper carbonate
(d) Carbonic acid
Answer:
(a) Sodium hydroxide
79. Which of the following is called alkali?
(a) Water soluble base
(b) Water insoluble base
(c) Carbonate of metals
(d) Oxides of metals
Answer:
(a) Water soluble base
80. What happens when an acid react with base?
(a) Acid neutralizes base
(b) Water is formed
(c) A salt is formed
(d) All of the above
Answer:
(d) All of the above
81. Which of the following is an olfactory indicator?
(a) Turmeric
(b) Onion
(c) Litmus
(d) All of the above
Answer:
(b) Onion
82. What happens when a base is added to vanilla?
(a) Colour of vanilla changes into red
(b) Vanilla becomes colourless
(c) Vanilla loses its smell
(d) Nothing happens
Answer:
(c) Vanilla loses its smell
83.What happens when an acid is added to vanilla?
(a) Colour of vanilla changes into red
(b) Vanilla becomes colourless
(c) Vanilla loses its smell
(d) Nothing happen
Answer:
(d) Nothing happen
84. What is the original colour of phenolphthalein solution which is an indicator?
(a) Colourless
(b) Red
(c) Pink
(d) Violet
Answer:
(a) Colourless
85. Phenolphthalein exhibits which colour with a base?
(a) Remains colourless
(b) Pink
(c) Red
(d) Green
Answer:
(b) Pink
86. Phenolphthalein exhibits which colour with an acid?
(a) Remains colourless
(b) Pink
(c) Red
(d) Green
Answer:
(a) Remains colourless
87. Methyl orange which is an indicator turns into which colour with an acid?
(a) Red
(b) Yellow
(c) Pink
(d) No colour
Answer:
(a) Red
88. Methyl orange which is an indicator turns into which colour with a base?
(a) Red
(b) Yellow
(c) Pink
(d) No colour
Answer:
(b) Yellow
89. What the original colour of methyl orange solution which is an indicator?
(a) Yellow
(b) Orange
(c) Pink
(d) Red
Answer:
(b) Orange
90. Which of the following compound is formed when zinc reacts with hydrochloric acid?
(a) Zinc sulphate
(b) Zinc chloride
(c) Zinc carbonate
(d) Zinc hydroxide
Answer:
(b) Zinc chloride
91. Which of the following compound is formed when zinc reacts with sodium hydroxide?
(a) Zinc hydroxide
(b) Sodium zincate
(c) Zinc hydrogenate
(d) No reaction takes place
Answer:
(b) Sodium zincate
92. Which of the following gas is formed when an acid reacts with metal carbonate?
(a) Carbon monoxide
(b) Carbonic acid gas
(c) Carbon dioxide gas
(d) Hydrochloric acid gas
Answer:
(c) Carbon dioxide gas
93. What happens when hydrogen carbonate reacts with an acid?
(a) Carbon monoxide
(b) Carbonic acid gas
(c) Carbon dioxide gas
(d) Hydrochloric acid gas
Answer:
(c) Carbon dioxide gas
94. What happens when carbon dioxide gas is passed through lime water?
(a) Lime water turns milky
(b) Lime water turns colourless
(c) Lime water turns bluish
(d) Lime water turns black
Answer:
(a) Lime water turns milky
95. What happens when excess of carbon dioxide gas is passed through lime water?
(a) Lime water first turns milky and then colourless
(b) Lime water turns bluish
(c) Lime water turns milky
(d) Lime water turns blackish
Answer:
(a) Lime water first turns milky and then colourless
96. What is the nature of non-metallic oxides?
(a) Basic
(b) Acidic
(c) Neutral
(d) None of the above
Answer:
(b) Acidic
97. A basic solution is added to a test tube. A blue and red litmus paper is dipped into the basic solution. What will happen to both litmus papers?
(a) Blue litmus paper: changes colour, red litmus paper, no colour change
(b) Blue litmus paper: changes colour, red litmus paper: changes colour
(c) Blue litmus paper: no colour change; red litmus paper changes colour
(d) Blue litmus paper no colour change, red litmus paper: no colour change
Answer:
(c) Blue litmus paper: no colour change; red litmus paper changes colour
98. A solution of pH 2 is filled in two separate beakers. A few drops of methyl orange and phenolphthalein are added into separate solutions. How will the colour of the indicators change?
(a) Methyl orange: red; phenolphthalein: pink
(b) Methyl orange: orange; phenolphthalein: colourless
(C) Methyl orange: red; phenolphthalein: colourless
(d) Methyl orange orange: phenolphthalein: pink
Answer:
(c) Methyl orange: red; phenolphthalein: colourless
99. When dilute sulphuric acid is added to a solid X, a gas Y is formed along with the formation of the salt of the solid. What could be X and Y?
(a) X: carbon: Y: hydrogen
(b) X: zinc, Y: hydrogen
(c) X: zinc, Y oxygen
(d) X: copper: Y: oxygen
Answer:
(b) X: zinc, Y: hydrogen
100. When a base reacts with a metal, it forms a salt and hydrogen gas is released By what method the presence of hydrogen can be detected?
(a) By methyl orange
(b) By water
(c) By litmus paper
(d) By a burning candle
Answer:
(d) By a burning candle