CLASS-X
CHAPTER -4 CARBON AND ITS COMPOUNDS
CARBON: Carbon is an element( Non Metal).
SYMBOL: C
ATOMIC NUMBER: 6
ELECTRONIC CONFIGURATION: 2,4
VALANCE ELECTRONS: 4
VALANCY: 4
ATOMIC MASS: 12
ISOTOPS: 6C12, 6C13 and 6C14
ALLOTROPS: Diamond, Graphite and fullerenes.
OCCURRENCE OF CARBON
1. Carbon is found I the atmosphere, inside the earth’s crust and in all living organisms. Carbon is present in fuels like wood, coal, charcoal , coke, petroleum, natural gas, biogas etc.
2. Carbon is present in compounds like carbonates and hydrogen carbonates.
3.Carbon is also found in free state as Diamond, Graphite and Fullerenes.
BONDING IN CARBON [Covalent bonds]
The atomic number of carbon is 6, its electronic configuration is 2,4. It has 4 valance electrons. It can attain the stability by gaining 4 electrons, by losing 4 electrons or by sharing 4 electrons with other atoms.
1. It does not gain 4 electrons because it is difficult for the 6 protons to hold the charge of 10 electrons.
2.It does not loss 4 electrons because it needs a large amount energy to lose 4 electrons.
3.Therefore, it share 4 electrons with other atoms to attain stability resulting in the formation of covalent bonds.
Note: Since, Carbon atom needs 4 electrons to attain stability, therefore its valency is 4 and it is tetravalent.
COVALENT BONDS
The bonds formed by the sharing of electrons between atoms are called covalent bonds. There are three types of covalent bonds on the basis of the number of electron pair to be shared.
(a)Single Covalent bond
(b)Double Covalent bond
(c)Triple covalent bond
(a)SINGLE COVALENT BONDS: The sharing of one pair of electrons between two atoms (of same or different element) resulting in the formation of single covalent bond.
Eg: Formation of single covalent bond in Hydrogen molecule(H2)
Hydrogen: H
Atomic no: 1
Electronic configuration: 1
Valance electrons: 1
![]()
(b)DOUBLE COVALENT BONDS: The sharing of two pairs of electrons between two atoms (of same or different element) resulting in the formation of double covalent bond.
Eg: Formation of double covalent bond in Oxygen molecule(O2)
Oxygen : O
Atomic no: 8
Electronic configuration: 2,6
Valance electrons: 6
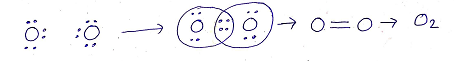
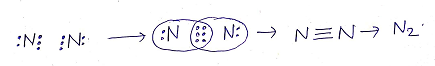
(c)TRIPLE COVALENT BONDS: The sharing of three pairs of electrons between two atoms (of same or different element) resulting in the formation of triple covalent bond.
Eg: Formation of triple covalent bond in Nitrogen molecule(N2)
Q: Draw the electron dot structure for the following compounds
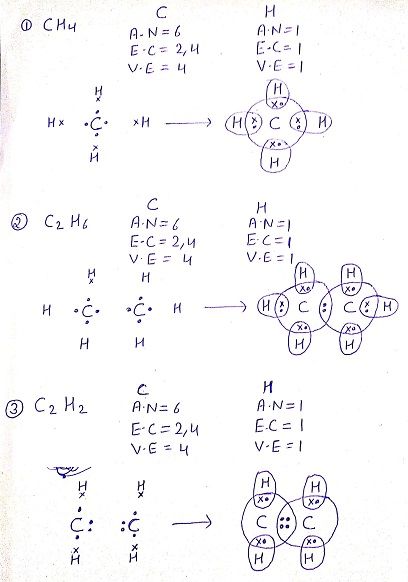
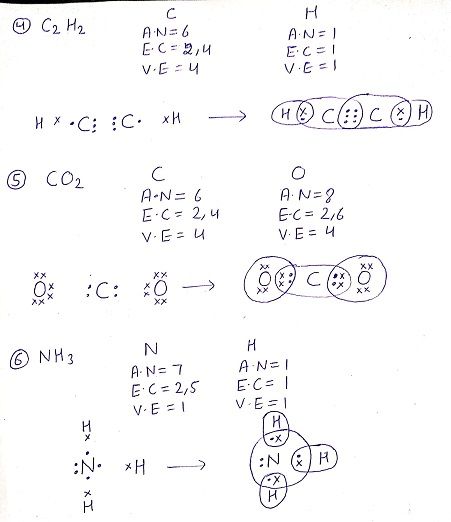
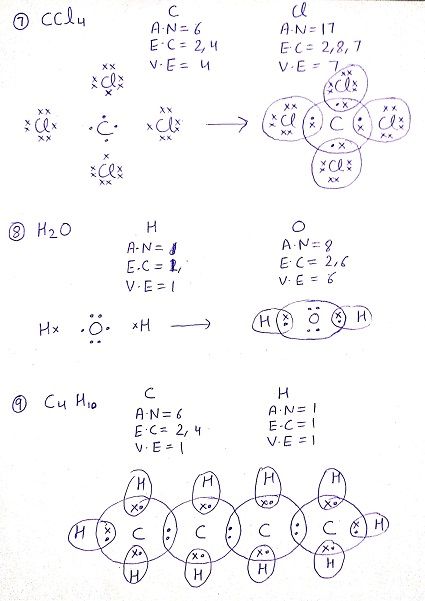
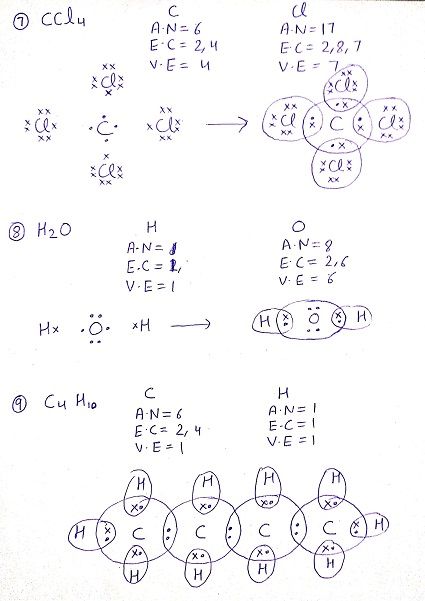
Q:What do you understand by versatile nature of carbon?
Or
Why carbon form a very large no of carbon compounds?
Ans: The number of carbon compounds is more than 3 millions. It is more than the number of compounds formed by all other elements. This is due to the following reasons.
1. Carbon atom can form bonds with other carbon atoms to form long chain, branched chain or closed ring. This property of carbon is called catenation.
2. Since the valancy of carbon is 4, therefore it can form bond with other 4 carbon atoms or with atoms of different elements like hydrogen , nitrogen, chlorine , fluorine . bromine etc.
ALLOTROPES :
Allotropes are the different forms of an element. Which has different physical properties or structure but have same chemical properties. An element can have more than one different forms.
ALLOTROPES OF CARBON: Carbon exist in different allotropic forms some of them are
1.CRYSTALLINE FORM: Diamond, Graphite and Fullerenes.
2.NON-CRYSTALLINE FORM: Coal, Charcoal and Lampblack.
DIAMOND
(a) Structure of Diamond:
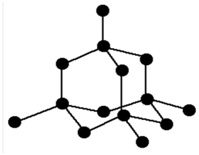
Structure of diamond
In the structure of diamond each carbon atom is bonded with other 4 carbon atoms by strong covalent bonds to form a three dimensional structure called Tetrahedral Structure. There is no free electron to conduct electricity. Therefore , diamond is a bad conductor of electricity. Strong covalent bonds between the carbon atoms makes it the world hardest substance.
(b) Properties of Diamond: Properties of diamond are-
1.Diamond is a transparent and colorless substances with extra ordinary shine due to its high refractive index.
2.It is the hardest natural substance due to strong covalent bonds and tetrahedral structure.
3.It is a bad conductor of electricity due to absence of free electrons
4.It has high melting and boiling point.
5.It burns on strong heating to form carbon di-oxide gas.
(c)Uses of diamond: The properties of diamond are-
1.Diamond is used as cutting instrument like glass cutter, marble cutter etc.
2.It is also used in ornaments or jewellery
3.It is also used in dies which are used for drawing wires from metals.
4.Diamond absorbs harmful radiation. Hence it is used in space satellite to make radiation proof window.
Q: How diamond can be prepared artificially?
Ans: Diamond can be prepared artificially by subjecting pure carbon to very high pressure and temperature. These synthetic diamonds are small but are indistinguishable from natural diamond.
GRAPHITE
(a)STRUCTURE OF GRAPHITE
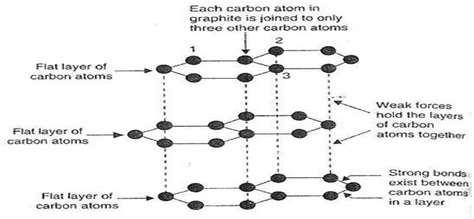
In the structure of Graphite, each carbon atom is bonded with other three carbon atoms to form hexagonal rings. These hexagonal rings combined to form a flat layer(sheet) of carbon. These layers of carbon atoms are held together by weak vander Waal’s force. So that these layers can slide over one another.
Since in the structure of graphite each carbon atom is bonded with other three carbon atoms. Therefore one electron remain free to conduct electricity. Hence it is a good conductor of electricity.
(b) PROPERTIES OF GRAPHITE
1.It is grayish black opaque substance.
2.It is lighter than diamond.
3.It is slippery to touch.
4.It is a good conductor of electricity but bad conductor of heat.
5.On strong heating, it burns to give CO2.
(c) USES OF GRAPHITE
1. Graphite is used as solid lubricant in electric motors.
2. it is used in the lid of pencil.
3.Its rods are used in nuclear reactor to absorbs excess neutrons.
4.It is used as electrode in electrolysis.
5. It can be used to make graphene sheets. Theses sheets are said to be 100 times stronger and 10 times lighter than steel.
FULLERENES
Fullerenes are the recently discovered allotrophic form of carbon which were prepared by H.W Kroto, Smalley and Robert Curt by the action of laser beam on the vapours of graphite.
Most commonly known fullerene contain 60 carbon atoms and known as Buckminster fullerene. Fullerene(C60) was named after the American architect Buckminster fuller because its structure resembled with the framework of dome shaped hall designed by fuller for the large international conference.
(a) STRUCTURE OF FULLERENE(C60 or Buckminster fullerene)
Structure of Buckminster fullerene(C60)
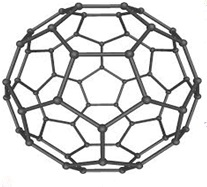
Buckminster fullerene
Fullerene (C60) is a football shaped spherical molecule in which 60 Carbon atoms are arranged in the form of interlinking hexagonal and pentagonal rings of carbon atoms. One C60 molecule contain 20 hexagonal rings and 12 pentagonal rings of carbon atoms.
(b)PRPERTIES OF FULLERENE
1. Fullerenes are dark solid at room temperature.
2.Fullerenes are neither too hard and nor too soft.
3.On burning, it gives carbon dioxide.
(c) USES OF FULLERENES
1.In pure form it behaves as insulator. However, it can be converted into semiconductors under suitable condition.
2. C60 molecule is a good absorber of oxygen atoms. Therefore , it is used in cancer as well as AIDS therapy.
3.These are used as catalysts in photochemical refining in industries.
Q:What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?(NCERT)
Q: What would be the electron dot structure of Carbon dioxide which has a molecular formula CO2?(NCERT )
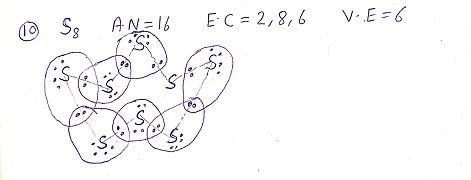
Q: What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? ( NCERT)
Q: What will be the formula and electron dot structure of cyclopentane? ( NCERT)
HYDROCARBONS
The compounds containing carbon and hydrogen atoms are called hydrocarbons. There are two types of hydrocarbons:
1.Saturated Hydrocarbons( single bond)
2.Unsaturated Hydrocarbons( at least one double or triple bond)
1.SATURATED HYDROCARBONS
In saturated hydrocarbons all the carbon atoms are bonded with single covalent bond.
Eg: Alkane: Alkanes have all single covalent bonds between carbon atoms and their names end with ‘ane’.
(i) Methane -CH4
(ii) Ethane -C2H6
(iii) Propane -C3H8
(iv) Butane -C4H10
2.UNSATURATED HYDROCARBONS
In unsaturated hydrocarbons at least two or more carbon atoms are bonded with double or triple bond.
Eg: Alkenes and Alkynes
Alkenes: Alkenes have at least one double covalent bond between two carbon atoms and their names end with ‘ene’.
(i) Ethene – C2H4
(ii) Propene -C3H6
(iii) Butene -C4H8
Alkynes: Alkynes have at least one double covalent bond between two carbon atoms and their names end with ‘yne’.
(i) Ethyne – C2H2
(ii) Propyne -C3H4
(iii) Butyne -C4H6
CHEMICAL PROPERTIES OF CARBON COMPOUNDS
(a) CUMBUSTION: Carbon compounds burn in oxygen to form water, carbon dioxide, heat and light.
Eg: (i)
C + O2 ——> CO2 + Heat + Light
(ii)
CH4 + 2O2 ———–> CO2 + 2H2O + Heat +Light
(iii)
C2H5OH + 3 O2——-> 2CO2 + 3H2O + Heat + Light
(b) ADDITION REACTION: Unsaturated hydrocarbons undergoes addition reaction with hydrogen in the presence of nickel and palladium as catalysts to form saturated hydrocarbons.
Eg: Ethene undergoes addition reaction with hydrogen to form ethane in the presence of nickel and palladium as catalyst.
Ni/Pd
C2H4 ——— ->C2H6
Ethene Ethane
Note: The addition of hydrogen to unsaturated hydrocarbon to form saturated hydrocarbons is called hydrogenation. Hydrogenation is used to convert unsaturated oils and fats to saturated oils and fats.
(c) SUBSTITUTION REACTION: Saturated hydrocarbons undergo substitution reaction with halogens to form substitutional products.
Eg:Methane undergoes substitution reaction with chlorine in the presence of sunlight to form substitutional products.
CH4 + Cl2———–> CH3Cl + HCl
CH3Cl + Cl2 ———–>CH2Cl2 + HCl
CH2Cl2 + Cl2 ———>CHCI3 + HCl
CHCI3 + Cl2 ——–> CCl4 + HCl
(d)OXIDATION: Carbon compounds like alcohols are oxidized to carboxylic acids on heating with oxidizing agents like alkaline potassium permanganate (KMnO4) or acisic potassium dichromate(K2Cr2O7).
(i) Alcohols are oxidized in carboxylic acids
Alkaline KMnO4/ acidic K2Cr2O7
Alcohol —————————–> Carboxylic acids
Alkaline KMnO4/ acidic K2Cr2O7
C2H5OH —————————–> CH3COOH
SOME IMPORTANT CARBON COMPOUNDS
a) ETHANOL :- C2H5OH – Ethyl alcohol
Properties :-
i) Ethanol is a colourless liquid with a pleasant smell and burning taste.
ii) It is soluble in water.
iii) Ethanol reacts with sodium to form sodium ethanoate and hydrogen.
2C2H5OH + 2Na ———–> 2C2H5ONa + H2
iv) Ethanol reacts with hot conc. H2SO4 to form ethene and water. Conc. H2SO4 is a dehydrating agent and removes water from ethanol.
conc. H2SO4
C2H5OH —————> C2H4 + H2O
Uses :-
i) Ethanol is used for making alcoholic drinks.
ii) It is used as a solvent.
iii) It is used for making medicines like tincture iodine, cough syrups, tonics etc.
b) ETHANOIC ACID :- CH3COOH – Acetic acid
Properties :-
i) Ethanoic acid is a colourless liquid with a pungent smell and sour taste.
ii) It is soluble in water.
iii) A solution of 5% to 8% ethanoic acid in water is called Vinegar.
iv) Esterification :-
Ethanoic acid reacts with ethanol to form the ester ethyl ethanoate in the presence of conc. H2SO4.
conc.H2SO4
CH3COOH + C2H5OH ————> CH3COOC2H5 + H2O
The reaction between carboxylic acid and alcohol to form an ester is called esterification.
v) Saponification :-
When an ester reacts with sodium hydroxide solution, the sodium salt of the carboxylic acid and the parent alcohol are formed. This reaction is called saponification.
Eg :-Ethyl ethanoate reacts with sodium hydroxide to form sodium acetate and ethanol.
CH3COOC2H5 + NaOH ———-> CH3COONa + C2H5OH
vi) Ethanoic acid reacts with bases to form salt and water.
CH3COOH + NaOH ———-> CH3COONa + H2O
vii) Ethanoic acid reacts with carbonates and hydrogen carbonates to form salt, water and carbon dioxide.
2CH3COOH + Na2CO3 ——-> 2CH3COONa + H2O + CO2
CH3COOH + NaHCO3 ———> CH3COONa + H2O + CO2
SOAPS AND DETERGENTS
(a) SOAPS: Soaps are long chain sodium or potassium salts of carboxylic acids. Eg:- Sodium stearate – C17H35COONa
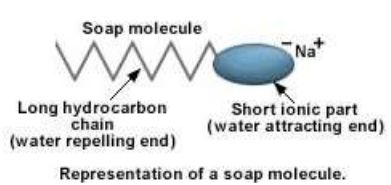
Structure of soap molecule:
A soap molecule has two parts. A long hydrocarbon part which is hydrophobic (water repelling) and soluble in oil and grease and a short ionic part which is hydrophyllic (water attracting) and insoluble in oil and grease.
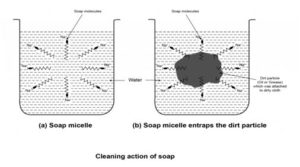
Cleansing action of soap :
When soap is dissolved in water it forms spherical structures called micelles. In each micelle the soap molecules are arranged radially such that the HC part is towards the centre and the ionic parts towards the outside. The HC part dissolves the dirt, oil and grease and forms an emulsion at the centre of the micelles which can be washed away by water.
 (b)DETERGENTS
(b)DETERGENTS
Detergents are long chain sodium salts of sulphonic acids. Soaps do not wash well with hard water because it forms insoluble precipitates of calcium and magnesium salts in hard water. Detergents wash well with hard water because it does not form insoluble precipitates of calcium and magnesium salts in hard water.
Q: Differentiate between Soap and Detergent.
Ans:
Soaps | Detergents |
1. Soaps are sodium salts of fatty acids. | 1. Detergents are sodium salts of sulphonic acids. |
2. Soaps clean well in soft water but do not clean well in hard water. | 2. Detergents clean well with both hard and soft water. |
3. Soaps do not clean as well as detergents. | 3. Detergents clean better than soaps. |
4. Soaps are biodegradable and do not cause pollution. | 4.Some detergents are non biodegradable and cause pollution |
Q: Why soap does not work with hard water?
Ans: Hard water contains calcium and magnesium ions which react with soap and form scum(insoluble substance) therefore soap does not work with hard water. On the other hand detergents do not form scum (insoluble precipitate) with calcium and magnesium ions in hard water. Thus, they remain effective in hard water.